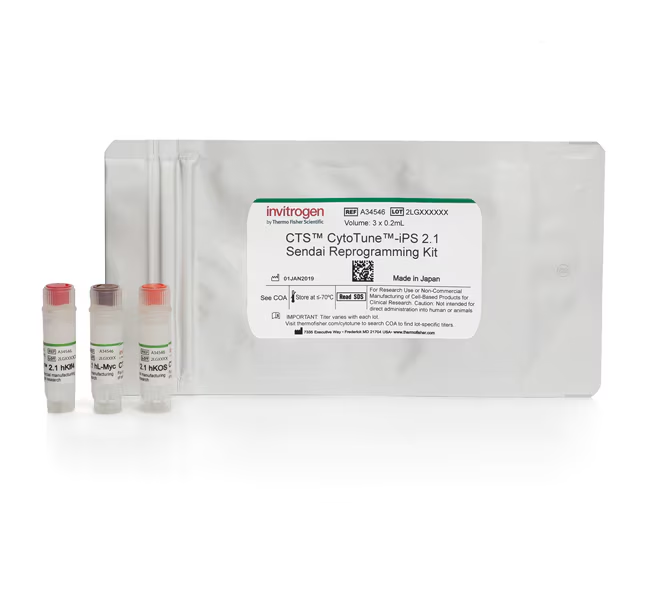
The CTS CytoTune-iPS 2.1 Sendai Reprogramming Kit is the first off-the-shelf reprogramming system designed for clinical and translational research. Like the CytoTune-iPS 2.0 Sendai reprogramming kit, this kit uses Sendai particles to deliver Yamanaka factors that are critical for efficient generation of induced pluripotent stem cells (iPSCs). This kit includes three vector preparations: polycistronic Klf4–Oct3/4–Sox2 (KOS), L-Myc, and Klf4.
Manufactured in accordance with GMP requirements
This product is manufactured at a site with a process that was audited for conformity with current Good Manufacturing Practices for medical devices, 21 CFR Part 820 of the regulation.
Formulated animal origin–free
The bovine serum albumin carrier protein was removed from the CTS CytoTune-iPS 2.1 Sendai vector formulation. The preparation contains no animal-derived components at the primary component level.
Accompanied by documentation to support your regulatory filing
Full traceability documentation such as Certificate of Origin and Certificate of Analysis are available.
What you get
The kit includes 0.2 mL of each vector (KOS, Klf4, and L-myc) at a titer of 0.5–2.0 x 108 CFU/mL, sufficient to reprogram a minimum of six wells of a six-well plate.
| Code | Description |
|---|---|
| A34546 | Catalog Number: A34546 |